New Surface Coating for Medical Devices Prevents Hospital Infections
New Surface Coating for Medical Devices Prevents Hospital Infections
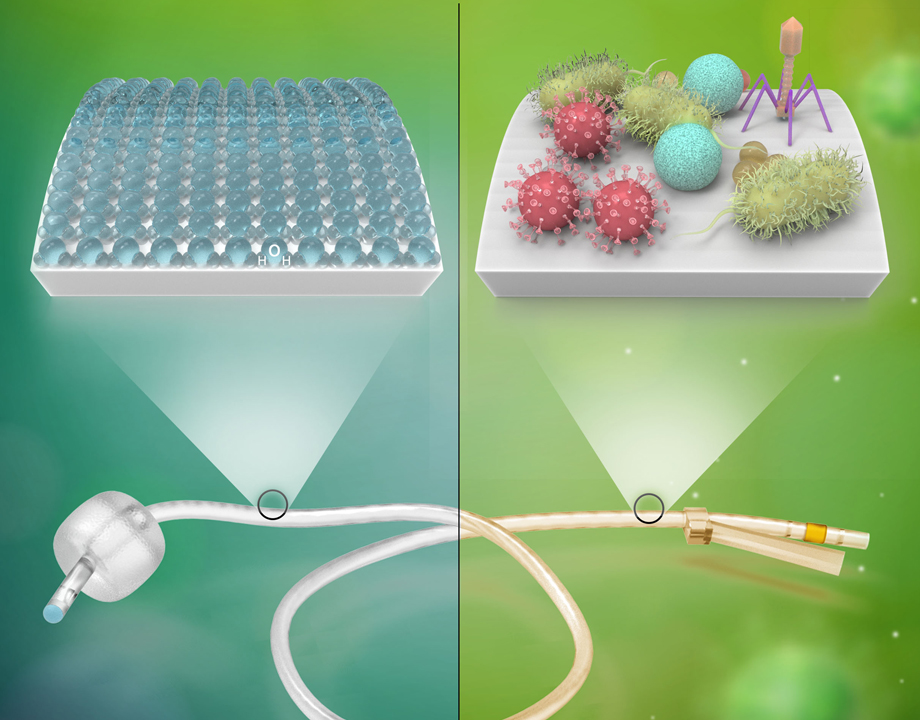

UCLA researchers develop a protective surface for medical devices made from zwitterionic polymers.
According to the Centers for Disease Control, patients in the U.S. contract about 1.7 million hospital-acquired infections every year, resulting in nearly 100,000 deaths, a multi-billion dollar economic burden, and widespread use of antibiotic drugs. As these stronger bacteria evolve, antibiotics become less effective, making these infections harder to treat.
The majority of these infections are derived from medical devices like catheters, stents, heart valves and pacemakers, whose surfaces often become covered with harmful bacterial films that lead to infection.
In an effort to improve this situation, Richard Kaner, chemistry professor and Ki Hong Professor of Materials Innovation at UCLA, and Brian McVerry, then-UCLA doctoral student and currently chief technology officer for their spin-off company, Silq Technologies Corporation, assembled a research team with the goal of finding a better way to reduce infection rates, improve the patient experience, and reduce operating costs for healthcare systems.
The team focused on developing a surface treatment for medical devices that resists bacteria and microbe growth, without relying on antibiotics.
For McVerry, the “aha” moment came when he was reading about materials that resist the deposition of organic material to surfaces called zwitterions, polymers with both positive and negative charges incorporated into their structure. “I wondered why the material was not in widespread use,” said McVerry.
Watch Our Video: Bioinspired Breakthroughs Transform Medical Devices
A literature review revealed that, although many academics were successful in depositing thin films of zwitterionic materials on surfaces, these processes required exotic reaction conditions, harsh chemical treatments, or expensive reagents, all of which were impossible to scale commercially.
“The question then became, how do we deposit these promising materials on a substrate, under ambient conditions, to leverage the zwitterionic materials resistance to adhesion?” asked McVerry.
Zwitterion polymers are highly biocompatible and absorb water very tightly, forming a thin hydration barrier that prevents bacteria and other micro-organisms from adhering to surfaces.
The team embarked on developing a one-step zwitterionic surface modification process. The biggest research challenge was developing a new material system that could scalably deposit zwitterionic materials on surfaces, under the following constraints:
In the lab, the zwitterion-based coating proved to be highly effective in reducing microbial adhesion to a variety of synthetic materials, including several clinically relevant materials, such as elastomeric polydimethylsiloxane. The treated surfaces showed robust adhesion resistance in both static and flow conditions.
More for You: Implanted Medical Devices Depend on Bioresorbable Materials
To test the clinical efficacy of this novel approach in a real-world clinical setting, a commercial silicone foley catheter treated with this material (and cleared by the Federal Drug Administration) was provided to 16 long-term catheterized patients. Silicone was a good choice for this test because, although it is a highly popular and useful biomedical material, its inherent tackiness attracts dirt and oils in the environment and bacteria and fungus when placed in the body.
Later, when they filled out a Patient Global Impression of Improvement (PGI-I) questionnaire, 10 out of the 16 patients described their urinary tract condition post-implantation as “much better” or “very much better” and 72 percent of patients decided to continue using the surface-treated catheter over conventional latex or silicone catheters.
The team claims the new zwitterion-based surface treatment is highly effective, nontoxic, and relatively low in cost compared with other current surface treatments for medical devices, such as antibiotic- or silver-infused coatings.
The work has possibilities to transform the surfaces of materials for applications outside the medical field. Possible applications include extending the lifetime of water-treatment devices and improving lithium-ion battery performance.
Take Our Quiz: Medical Device Evolution
“We have demonstrated that we can make otherwise tacky materials, like rubber, non-tacky,” said McVerry. “And modifications to the surface treatment can help materials glide across each other with less friction.”
The next step is commercialization.
“Several top companies have contacted us in different fields and have expressed desire to apply our surface treatment technology to their devices to reduce complications,” said McVerry. “Our research will continue to build on our work and demonstrate reduction in the foreign-body response and thrombosis on treated devices.”
Mark Crawford is a science and technology writer in Corrales, N.M.
The majority of these infections are derived from medical devices like catheters, stents, heart valves and pacemakers, whose surfaces often become covered with harmful bacterial films that lead to infection.
In an effort to improve this situation, Richard Kaner, chemistry professor and Ki Hong Professor of Materials Innovation at UCLA, and Brian McVerry, then-UCLA doctoral student and currently chief technology officer for their spin-off company, Silq Technologies Corporation, assembled a research team with the goal of finding a better way to reduce infection rates, improve the patient experience, and reduce operating costs for healthcare systems.
The team focused on developing a surface treatment for medical devices that resists bacteria and microbe growth, without relying on antibiotics.
For McVerry, the “aha” moment came when he was reading about materials that resist the deposition of organic material to surfaces called zwitterions, polymers with both positive and negative charges incorporated into their structure. “I wondered why the material was not in widespread use,” said McVerry.
Watch Our Video: Bioinspired Breakthroughs Transform Medical Devices
A literature review revealed that, although many academics were successful in depositing thin films of zwitterionic materials on surfaces, these processes required exotic reaction conditions, harsh chemical treatments, or expensive reagents, all of which were impossible to scale commercially.
“The question then became, how do we deposit these promising materials on a substrate, under ambient conditions, to leverage the zwitterionic materials resistance to adhesion?” asked McVerry.
Zwitterion polymers are highly biocompatible and absorb water very tightly, forming a thin hydration barrier that prevents bacteria and other micro-organisms from adhering to surfaces.
The team embarked on developing a one-step zwitterionic surface modification process. The biggest research challenge was developing a new material system that could scalably deposit zwitterionic materials on surfaces, under the following constraints:
- The treatment chemistry must be able to permanently bind to clinically relevant plastics and rubbers.
- The surface treatment must be nontoxic.
- The treatment must be applied rapidly under ambient conditions to maintain the scale of medical device manufacturing.
- The treatment must not incorporate antibiotics to maintain global antimicrobial stewardship efforts.
In the lab, the zwitterion-based coating proved to be highly effective in reducing microbial adhesion to a variety of synthetic materials, including several clinically relevant materials, such as elastomeric polydimethylsiloxane. The treated surfaces showed robust adhesion resistance in both static and flow conditions.
More for You: Implanted Medical Devices Depend on Bioresorbable Materials
To test the clinical efficacy of this novel approach in a real-world clinical setting, a commercial silicone foley catheter treated with this material (and cleared by the Federal Drug Administration) was provided to 16 long-term catheterized patients. Silicone was a good choice for this test because, although it is a highly popular and useful biomedical material, its inherent tackiness attracts dirt and oils in the environment and bacteria and fungus when placed in the body.
Later, when they filled out a Patient Global Impression of Improvement (PGI-I) questionnaire, 10 out of the 16 patients described their urinary tract condition post-implantation as “much better” or “very much better” and 72 percent of patients decided to continue using the surface-treated catheter over conventional latex or silicone catheters.
The team claims the new zwitterion-based surface treatment is highly effective, nontoxic, and relatively low in cost compared with other current surface treatments for medical devices, such as antibiotic- or silver-infused coatings.
The work has possibilities to transform the surfaces of materials for applications outside the medical field. Possible applications include extending the lifetime of water-treatment devices and improving lithium-ion battery performance.
Take Our Quiz: Medical Device Evolution
“We have demonstrated that we can make otherwise tacky materials, like rubber, non-tacky,” said McVerry. “And modifications to the surface treatment can help materials glide across each other with less friction.”
The next step is commercialization.
“Several top companies have contacted us in different fields and have expressed desire to apply our surface treatment technology to their devices to reduce complications,” said McVerry. “Our research will continue to build on our work and demonstrate reduction in the foreign-body response and thrombosis on treated devices.”
Mark Crawford is a science and technology writer in Corrales, N.M.





