Renewed Meniscus
Renewed Meniscus
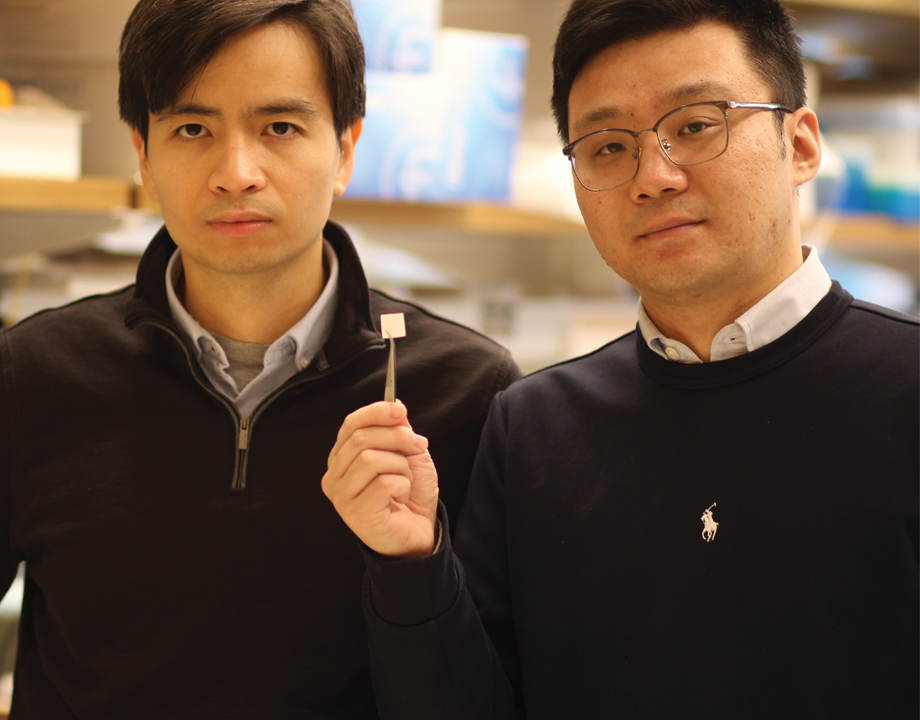

Thanh D. Nguyen and Yang Liu hold breakthrough tissue scaffold designed to regenerate cartilage. Photo: University of Connecticut
As we age, many of us will feel the occasional aches; however, this can be exacerbated with joint pain caused by cartilage deterioration due to injuries, sports, or diseases like osteoarthritis.
According to the latest statistics from the Centers for Disease Control, about one in four adults, or 58.5 million people, have been diagnosed with arthritis. This disease can severely limit mobility and has been the culprit behind working-age people unable to hold certain jobs.
“The tissue in the knee joint to cushion the bone when you are running or walking disappears over time until you get bone colliding with bone, which is really painful,” said Thanh D. Nguyen, an assistant professor of mechanical engineering at the University of Connecticut. Eventually, the entire knee must be replaced.
However, his researchers at the university are enjoying their first breakthrough to make joint pain a thing of the past.
Recently, the team watched its rabbit patient hop normally on a mini treadmill. The animal previously suffered from cartilage deterioration. Two months prior, Cato Laurencin, a professor and orthopedic surgeon at the university, successfully implanted a tissue scaffold into the rabbit’s knee in order to encourage the healthy cells in the knee to regenerate.
More for You: Antibacterial Copper to Heal Bones, Osteomyelitis
The team designed a tissue scaffold nanomaterial out of a biodegradable polymer used to suture wounds called piezoelectricity.
“This means when you apply force on the material, it produces an electrical charge. Like a battery — a very safe, biodegradable battery — that uses movement to produce the electrical cues that are very useful for tissue regeneration,” Nguyen said.
Currently, the best treatments for deteriorated cartilage include transplanting healthy cartilage from somewhere else in your own body, or using a donor. The former can compromise the place from where the healthy cartilage was taken, and the body could reject donor material. This makes complete knee replacements a go-to solution.
However, Nguyen said he was inspired to consider an alternative treatment when a friend gifted him a book.
“The book talks about the body as basically an electrical system. When there is a wound, the body produces a small electrical current in the wound itself,” he explained. “In the opposite way, you can use the electrical charge or signals to help regrow the tissue.”
This made him want to mimic the body’s natural process using a biodegradable material and electric currents. Nguyen and his team have been researching this process since 2015, said Yang Liu, a postdoctoral fellow and research team member.
What makes this breakthrough exciting is that once implanted within the joint, the material encourages the surrounding stem cells to regrow the cartilage using electrical charges. The material acts like a magnet drawing the appropriate stem cells onto it.
“This is a natural process,” Liu said. “The body’s movement produces an electrical charge, which is also mimicked through the biodegradable material. Both encourage the body’s natural healing process.”
Reader’s Choice: Lab-Grown Meat: A Big Step Forward
So, every time the rabbit exercised, the tissue in the knee joint sent an electric current to the surrounding cells encouraging them to regenerate. They did. The rabbit now has healthy knee cartilage that mimics the original materials once there.
“Technically, what we saw looked exactly like the native tissue,” Nguyen said of the regenerated cartilage. “It should last. In principle it should function like the native tissue. It’s not certain, because we don’t have the data yet. But, we will do a long-term study on this naturally healed tissue to confirm the hypothesis.”
The results were really amazing, he said.
Although it took two months for the cartilage to regenerate in the rabbit, which included one month of rest and one month of exercise, Liu said the process should be about three to six months for humans. The team hopes to go to human trials in the next two to three years. The process still needs to be successful and safe on a larger animal before achieving FDA approval.
What is also special about this breakthrough is that the tissue scaffolding can be used in other types of regeneration: wounds, nerve cells, skin, bone, and muscle injuries.
“All things can be stimulated by the electrical charge,” Nguyen emphasized. “How much? What’s the modality? We are still studying.”
In addition to the technology being used for regenerating cartilage, it’s also used to create a facemask filter product. “These masks are reusable and biodegradable. They perform even better than the N95 masks,” he claimed.
Editor’s Pick: Handheld Device May Stop Fatal Blood Loss
Nguyen’s company, PiezoBioMembrane, has already created a successful prototype. He hopes to join with a manufacturing company to mass produce this product. Ideally, the product’s success will aid with acquiring resources for other intensive applications like the cartilage scaffolds.
However, today, Nguyen and his team keep their eyes on the day when they can bring this new technology to the people who need it. Many like him and Liu have family and friends who suffer from joint pain.
Even now, Nguyen said, people email him daily about wanting to be part of the human trials. “It’s understandable,” he said of their eagerness. “Arthritis is very painful.”
Nichole M. Palmer is an independent writer in Charlotte, N.C.
According to the latest statistics from the Centers for Disease Control, about one in four adults, or 58.5 million people, have been diagnosed with arthritis. This disease can severely limit mobility and has been the culprit behind working-age people unable to hold certain jobs.
“The tissue in the knee joint to cushion the bone when you are running or walking disappears over time until you get bone colliding with bone, which is really painful,” said Thanh D. Nguyen, an assistant professor of mechanical engineering at the University of Connecticut. Eventually, the entire knee must be replaced.
However, his researchers at the university are enjoying their first breakthrough to make joint pain a thing of the past.
Recently, the team watched its rabbit patient hop normally on a mini treadmill. The animal previously suffered from cartilage deterioration. Two months prior, Cato Laurencin, a professor and orthopedic surgeon at the university, successfully implanted a tissue scaffold into the rabbit’s knee in order to encourage the healthy cells in the knee to regenerate.
More for You: Antibacterial Copper to Heal Bones, Osteomyelitis
The team designed a tissue scaffold nanomaterial out of a biodegradable polymer used to suture wounds called piezoelectricity.
“This means when you apply force on the material, it produces an electrical charge. Like a battery — a very safe, biodegradable battery — that uses movement to produce the electrical cues that are very useful for tissue regeneration,” Nguyen said.
Currently, the best treatments for deteriorated cartilage include transplanting healthy cartilage from somewhere else in your own body, or using a donor. The former can compromise the place from where the healthy cartilage was taken, and the body could reject donor material. This makes complete knee replacements a go-to solution.
However, Nguyen said he was inspired to consider an alternative treatment when a friend gifted him a book.
“The book talks about the body as basically an electrical system. When there is a wound, the body produces a small electrical current in the wound itself,” he explained. “In the opposite way, you can use the electrical charge or signals to help regrow the tissue.”
This made him want to mimic the body’s natural process using a biodegradable material and electric currents. Nguyen and his team have been researching this process since 2015, said Yang Liu, a postdoctoral fellow and research team member.
What makes this breakthrough exciting is that once implanted within the joint, the material encourages the surrounding stem cells to regrow the cartilage using electrical charges. The material acts like a magnet drawing the appropriate stem cells onto it.
“This is a natural process,” Liu said. “The body’s movement produces an electrical charge, which is also mimicked through the biodegradable material. Both encourage the body’s natural healing process.”
Reader’s Choice: Lab-Grown Meat: A Big Step Forward
So, every time the rabbit exercised, the tissue in the knee joint sent an electric current to the surrounding cells encouraging them to regenerate. They did. The rabbit now has healthy knee cartilage that mimics the original materials once there.
“Technically, what we saw looked exactly like the native tissue,” Nguyen said of the regenerated cartilage. “It should last. In principle it should function like the native tissue. It’s not certain, because we don’t have the data yet. But, we will do a long-term study on this naturally healed tissue to confirm the hypothesis.”
The results were really amazing, he said.
Although it took two months for the cartilage to regenerate in the rabbit, which included one month of rest and one month of exercise, Liu said the process should be about three to six months for humans. The team hopes to go to human trials in the next two to three years. The process still needs to be successful and safe on a larger animal before achieving FDA approval.
What is also special about this breakthrough is that the tissue scaffolding can be used in other types of regeneration: wounds, nerve cells, skin, bone, and muscle injuries.
“All things can be stimulated by the electrical charge,” Nguyen emphasized. “How much? What’s the modality? We are still studying.”
In addition to the technology being used for regenerating cartilage, it’s also used to create a facemask filter product. “These masks are reusable and biodegradable. They perform even better than the N95 masks,” he claimed.
Editor’s Pick: Handheld Device May Stop Fatal Blood Loss
Nguyen’s company, PiezoBioMembrane, has already created a successful prototype. He hopes to join with a manufacturing company to mass produce this product. Ideally, the product’s success will aid with acquiring resources for other intensive applications like the cartilage scaffolds.
However, today, Nguyen and his team keep their eyes on the day when they can bring this new technology to the people who need it. Many like him and Liu have family and friends who suffer from joint pain.
Even now, Nguyen said, people email him daily about wanting to be part of the human trials. “It’s understandable,” he said of their eagerness. “Arthritis is very painful.”
Nichole M. Palmer is an independent writer in Charlotte, N.C.



